Full Text View
GSA Today
Article, pp. 4-10 | Abstract | PDF (3.8MB)
The evolution of end-member continental waters: The origin of acidity in southern Western Australia
ABSTRACT
The Yilgarn Craton of Western Australia hosts a regional acid saline groundwater system and hundreds of ephemeral saline lakes characterized by complex acid brines. These acid saline lakes and groundwaters have pH as low as 1.4 and salinities as high as 32% total dissolved solids. The low pH formed by a combination of processes dependent upon the host rock lithology and mineralogy, climate, weathering, organisms, and time. Although these modern acid saline environments are relatively rare, they have both ancient terrestrial and extraterrestrial counterparts. Understanding acidification processes provides enhanced understanding of hydrosphere-lithosphere-atmosphere-biosphere interactions. These environments present evidence of new brine evolution pathways and suggest the potential for future intense acid brine environments.
*Email:
DOI: 10.1130/GSATG231A.1
Manuscript received 31 Aug. 2014; accepted 6 Jan. 2015.
Introduction
The “wheat belt” and “gold fields” of southern Western Australia are associated with a regional acid saline groundwater system. Groundwaters have pH as low as 2.4 and salinities as high as 28% total dissolved solids (TDS) and have greatly affected bedrock and subsurface sediments. The surface expressions of these acid brine groundwaters are the hundreds of shallow, ephemeral acid saline lakes with pH as low as 1.4 and salinities as high as 32% TDS (Fig. 1) (Benison et al., 2007; Bowen and Benison, 2009). These acid lake and groundwaters are chemically complex. They are rich in Na-Cl-Mg-SO4, poor in HCO3, and have unusually high and highly variable concentrations of Al, Si, Fe, Br, and some other metals (Bowen and Benison, 2009; Gray, 2001). The origin of the acidity is likely sulfuric acid, with subsequent production of hydrochloric and bromic acids (Benison and Bowen, 2013).
|
Yilgarn Craton in southern Western Australia. (A) Map of southern portion of Yilgarn Craton. Approximate locations and general pH range of 60 lakes studied in detail since 2001. (B) Image of “wheat belt” landscape near “gold fields,” located southwest of Salmon Gums. Note the many lakes with different colors. |
The acid saline lakes and groundwaters are hosted by Archean rocks of the Yilgarn Craton and a thin, laterally discontinuous cover of sediment and sedimentary rock. The Yilgarn Craton contains granite-gneiss complexes and greenstone belts, which are deformed, faulted, and show a range of metamorphic alteration and various degrees of physical and chemical weathering. These rocks are mined locally for gold, iron, nickel, copper, lead, zinc, aluminum, uranium, and rare earth elements, and some ore concentrations are related to migration of the acid brines (Lawrance, 2001). Archean outcrops are found under and adjacent to some lakes and in direct contact with some modern lake water. Localized “regolith” (i.e., highly weathered Archean rock) has preserved some igneous and metamorphic textures from the precursor rock. However, its mineralogy includes Fe-oxides, kaolinite, and quartz, which are suggestive of alteration by acid saline waters (Fig. 2) (Bowen et al., 2013). Thin Eocene–Quaternary sandstones, lignites, and rare carbonates are found up to 100 m deep in paleo-channel basins (Clarke, 1993, 1994; Clarke et al., 1996). Thin recent sandstones and ironstones are found along the shorelines of some acid saline lakes. Modern clastic sediments include quartz sand and reworked evaporites. Acid lake waters precipitate halite, gypsum, hematite, kaolinite/halloysite, and rare opaline silica (Fig. 2). Acid groundwaters precipitate a suite of displacive crystals and early cements in the surface and shallow subsurface sediments. These very early digenetic minerals include halite, gypsum, hematite, goethite, jarosite, alunite, rozenite, gibbsite, kaolinite/halloysite, dickite, and hydrobasaluminute (Fig. 2) (Benison et al., 2007; Bowen et al., 2012; Story et al., 2010).
|
Photos of acid brine-influenced landscapes, rocks, and sediments in southern Western Australia. (A) Highly weathered outcrop near Leonora. (B) Altered granite in Hyden containing yellow and orange jarosite iron oxide and sulfate minerals. (C) Dry lake and surrounding sand flat east of Norseman. (D) Acid brine lake with white halite and yellow water near Salmon Gums. (E) Cross section of shallow sand flat sediment adjacent to acid brine lake southwest of Norseman. Note mottles of iron oxides, jarosite, and alunite. (F) Cross section of sand dune composed of iron oxide–coated gypsum grains near Merredin. (G) Cross section of acid sulfate soil near Toodyay. (H) Cross section of iron oxide concretions in recent sandstone near Kellerberrin. |
Neutral saline lakes in southern Western Australia are the anomaly here (Fig. 1). Some also have adjacent neutral groundwaters and overlie paleochannels, suggesting buffering of regional acid groundwaters by rare Eocene limestones at depth. Other neutral lakes are underlain by acid or moderately acid groundwaters. The larger neutral lakes may have a greater ratio of meteoric runoff relative to acid groundwaters. Other neutral lake waters are separated by mud from acid ground-waters, effectively making the neutral lake a perched meteoric water table.
Acid saline groundwater is widespread and seeps into most lakes to contribute to their unusual chemistry. The overarching question is: How did the extreme acidity form here?
Background
Hundreds of ephemeral saline lakes with changing colors in southern Western Australia were noted by Gregory (1914). The acidity of these saline lakes was recognized by Mann (1983). McArthur et al. (1989, 1991) analyzed pH, salinity, and major and minor ions in acid saline lakes and groundwaters near Salmon Gums, in particular Lakes Gilmore and Swann. Alpers et al. (1992) reported stable isotopes from two of the same samples. More recent work illustrated the spatial and temporal complexity of these dynamic systems (Benison et al., 2007; Bowen and Benison, 2009).
In southern Western Australia, the acidity has been attributed to ferrolysis, weathering, and oxidation of Fe2+ (Gray, 2001; McArthur et al., 1989, 1991; Mann, 1983). The abundance of secondary iron oxides in the region points to the importance of iron redox cycling in this geochemical system (Anand and Paine, 2002). McArthur et al. (1991) suggested that ferrolysis is the main source of the acidity, mainly due to the high iron content in the lowest pH waters analyzed at that time. Alpers et al. (1992) and Long et al. (1992) called for sea spray aerosols as contributors to the chemistry of acid saline waters. Long and Lyons (1990) theorized that acid saline waters might be the natural late-stage product of continental evolution. If so, the recognition of other acid saline lake systems in the rock record may not only help us to interpret local and regional climate histories of the past, it can also help us to make interpretations about large-scale processes, such as tectonic evolution and continental weathering.
Sources of Acidity
The most likely initial, and perhaps most important, acidification process is the oxidation of sulfides. This interpretation is supported by the presence of sulfides in host rocks, high sulfur in waters, abundant sulfate minerals, and oxidizing environment (Benison and Bowen, 2013). We have observed sulfur veins in Archean greenstone rocks from outcrops and mine tailings near Norseman. We also have observed disseminated pyrite in felsic igneous and metamorphic rock cores from the subsurface near Kalgoorlie. Disseminated sulfides, including Fe- and Cu- sulfide minerals, are found in the Archean rocks (R. Whittem, 2006, pers. comm.). There is high sulfate content (up to 35,000 ppm) and excess sulfur in waters (more S than can be accounted for with sulfate), indicating the presence of other S species (Bowen and Benison, 2009). Acid lakes and adjacent environments are characterized by abundant sulfate minerals, including gypsum, jarosite, and alunite (Benison and Bowen, 2013; Benison et al., 2007). In addition, the byproducts of ferric- and copper-sulfide oxidation are found here, including high Fe and Cu in waters and iron oxide minerals (Bowen and Benison, 2009).
Secondary acidification processes that likely occur in southern Western Australia involve combined oxidation and hydrolysis. Ferrolysis is a combined process of oxidation and hydrolysis that occurs in waters enriched with dissolved iron and yields additional H+, causing water pH to decrease. Similar chemical reactions that influence pH occur with waters enriched with dissolved aluminum. Repeated precipitation and dissolution of hematite, jarosite, alunite, and gibbsite, as well as other Fe- and/or Al-bearing minerals, may provide varying concentrations of dissolved Fe and Al that can contribute to these pH-lowering reactions. Some lakes have low dissolved Fe and little Fe-minerals (Bowen and Benison, 2009), suggesting that ferrolysis is not as important as sulfide oxidation at these lakes.
Another secondary acidification process that likely occurs in southern Western Australia is due to acidophilic microorganisms. Macrofauna are noticeably absent from the acid saline lakes, and nearby vegetation is of low diversity, especially compared to nearby neutral saline lakes (Benison, 2008). However, evidence of microorganisms has been detected in the field (Benison, 2008). Molecular methods have documented diverse communities of prokaryotes in the acid saline lakes (Mormile et al., 2009). The majority of these prokaryotes are novel, but some of their closest matches are S- and Fe-oxidizing bacteria. Other microbiological studies of these acid saline lakes suggest the additional presence of eukaryotes, such as acidophilic and halophilic alga and fungi (Benison, 2012; S.S. Johnson, 2014, pers. comm.). In addition, microorganisms have been detected as solid inclusions and within fluid inclusions in halite and gypsum precipitated from these acid saline lakes (Benison et al., 2008; Conner and Benison, 2013). It is known that many acidophilic microorganisms can promote biochemical processes such as Fe- and S- oxidation, resulting in even lower pH (e.g., Langworthy, 1978; Oren, 2010). Although more work is needed to better understand the specific microbial–water geochemistry relationships in the Western Australia acid saline lakes, it is likely that the microorganisms are influencing the pH of the lakes and groundwaters.
Climate and weather also play a role in acidity. In the semi-arid climate of southern Western Australia, evaporation greatly effects lake waters and, to a lesser extent, shallow groundwaters. Evaporation drives off water from the acid saline brines, resulting in more concentrated liquids. This evapo-concentration decreases pH and increases salinity. During dry times, lake waters have pH levels ~1–2 units lower than when the lakes are flooded. Likewise, shallow groundwaters have their lowest pH when the lakes are desiccated and shallow groundwater is evaporating. We have observed these lowest pHs in lakes during late stages of evapo-concentration (Benison et al., 2007; Bowen and Benison, 2009). In addition, laboratory experiments show that pH of moderately acidic water decreases several pH units upon evaporation (Foster and Benison, 2006; Long et al., 1992).
Recycling of acid waters may occur as part of flooding–evapo-concentration–desiccation cycles (Lowenstein and Hardie, 1985; Benison et al., 2007). When halite and gypsum grow in the acid lakes, they trap abundant acid fluid inclusions, which may compose up to ~30%–40% by volume halite and ~10%–20% by volume gypsum. When lakes flood and the halite and gypsum dissolve (halite at a much greater rate than gypsum), the acid fluid inclusions are released. Although this may be a small addition by volume to the water, it likely contributes to the lowering of the water pH after flooding.
The high degree of weathering of Archean rocks of the Yilgarn Craton has resulted in little buffering capacity. The result may be the limited presence of geochemical “buffers,” such as carbonate minerals and feldspars (Bowen et al., 2013; Long and Lyons, 1990, 1992). This has allowed low pH produced by acidification processes to be maintained.
The extensive weathering of Archean rocks not only limits buffering capacity but also may yield other acids. For example, chemical weathering dissolves many minerals and yield waters rich in ions, such as Cl, Br, and Fe. The high amounts of Cl− and Br− in the acid waters suggest that hydrochloric and bromic acids exist here in addition to sulfuric acid.
Expanding the Brine Evolution Model
Hardie and Eugster (1970) and Eugster (1970) pioneered the use of evaporite minerals to trace past brine evolution and the history of evaporite sediments in closed basins. This brine evolution model has evolved through the decades to incorporate the details of chemical pathways for various neutral-alkaline brines, many of which include carbon species and carbonate minerals as important players (e.g., Eugster, 1980; Eugster and Hardie, 1978; Eugster and Jones, 1979) (Fig. 3). Long et al. (2009) added the first low pH branch to the geochemical model (Fig. 4). There are likely variations of acid brine evolution, just as variations in neutral and alkaline brines exist. More work from the range of different acid brine environments is required to build a brine evolution model that fully encompasses the compositional and mineralogical range produced by natural brines.
|
Ternary diagram in equivalents for Ca-SO4-HCO3 showing the dominant carbonate and sulfate chemical divides that commonly occur as water evaporates. These types of diagrams are typically used to describe brine evolution in alkaline, neutral, or Ca-Cl type waters. The lack of HCO3− and calcite in the Western Australia (WA) acid lakes demonstrates that these systems are endmembers. WA lake waters are shown as orange squares and WA groundwaters are red circles (Bowen and Benison, 2009). Typical WA rain water values after Hingston and Gailitis (1976). Other examples of global extreme endmember saline systems are illustrated in comparison to the WA system. 1–3: Spring waters from China; 4–11: Surface brines from China lakes; average seawater and average river water; all after Lowenstein et al. (1989); 12: Salton Sea; 13: Great Salt Lake; 14: Deep Springs Lake; 15: Walker Lake; 16: Mono Lake; 17: Pyramid Lake; all after Spencer (2000). |
|
Brine evolution model for closed basins, featuring the Long-Lyons-Hines (2009) model for acid saline Lake Tyrrell spring zone waters, and based on the Eugster-Hardie-Jones brine evolution concept (Eugster, 1980; Eugster and Hardie, 1978; Eugster and Jones, 1979). In this variation of the model, abundance of HCO3 determines starting path. Several evolution pathways have been documented for specific closed lakes with neutral alkaline conditions, especially those in Africa and the western U.S.; in a complete model, these would appear to the right of the detailed Lake Tyrrell path. The more extreme and variable acid brines in southern Western Australia would appear as several complex evolution pathways to the left of the Lake Tyrrell path. |
The Long-Lyons-Hines (2009) brine evolution model was based on the acid spring zones along the shorelines of Lake Tyrrell in northwestern Victoria. Their model suggests a geochemical evolution starting with waters enriched in Ca and Mg, but depleted in HCO3. These waters precipitate gypsum (CaSO4 • 2H2O) and halite (NaCl), undergo sulfide oxidation and ferrolysis to become acidified, and precipitate Fe-oxides, jarosite [KFe3(SO4)2(OH)6], and alunite [KAl3(SO4)2(OH)6]. The ending waters are Na-Mg-Cl-SO4 acid brines (Long et al., 2009) (Fig. 4). We note that the general water evolution is the same in southern Western Australia, as well as, perhaps, throughout the continent. However, the Yilgarn Craton acid brines present more complex compositions. For example, some lakes have Al + Si + Fe > Ca + Mg + K (Bowen and Benison, 2009). Some acid brines have unusually high Al and/or Si (up to 8,017 ppm Al and up to 13,300 ppm Si), but low Fe (0–10 ppm), and some have high Fe (up to 459 ppm), but lower Al and/or Si. There are also temporal and spatial variations in major ions at individual lakes. Because of this complexity and variability of water chemistry and mineral precipitation and dissolution, no one brine evolution pathway can be designated for the Western Australian acid brines (Fig. 4). Figure 5 presents a flow chart that depicts our general understanding of the geological and geochemical evolution for the Yilgarn Craton of Western Australia, based on observations published in Benison et al. (2007), Benison and Bowen (2013), Bowen and Benison (2009), and Bowen et al. (2013). We hypothesize that a transition from a warm and wet to a warm and dry climate promoted the early chemical weathering and late evaporation, oxidation, and acidification. Warm and wet weathering in the Tertiary would have greatly decreased the buffering minerals (Long and Lyons, 1992). Later arid climate would have decreased the water:rock ratio, as well as enhanced concentration of solutes, increasing the salinity. Laboratory experiments and field observations show that evapo-concentration decreases pH if the starting solution was moderately acid (Foster and Benison, 2006).
|
Flow chart showing proposed evolution of geology and geochemistry of acid brine lake waters and groundwaters in southern Western Australia. |
Maintenance of Acid Brine Environments
Geochemical cycling and physical reworking operate dynamically on both varying temporal and spatial scales on the Yilgarn Craton. An understanding of physical and chemical sedimentological processes is necessary for interpreting acid brine evolution. Flooding-evaporation-desiccation cycles controlled by local weather at individual acid saline lakes drive much of this recycling (Benison et al., 2007). For example, lake water may precipitate gypsum, halite, iron oxides, and kaolinite. When the lake dries up, winds can entrain and transport those chemical sediments, depositing them in the same lake or tens to hundreds of kilometers away. Flooding of a lake due to a rainstorm will carry sediments in sheet floods to lakes from dunes, soils, and sandflats. In addition, the meteoric water may dissolve some soluble minerals, such as halite and gypsum, releasing their ions back into the lake water. These dynamic surface processes cause the lake waters to fluctuate in pH and salinity, thus crossing geochemical divides that determine precipitation versus dissolution of specific minerals. Geochemical cycling of sulfur, as well as other elements, particularly Cl, Fe, and Al, is intense (Benison and Bowen, 2013). Changes in dissolved Fe and S, in particular, play a role in keeping the pH low. The acid brine lake water–groundwater systems seem to be maintaining themselves by these processes.
Widespread Acid Brines Throughout Australia
Acid sulfate minerals, soils, and weathering profiles have been reported across Australia. Lake Tyrrell in northern Victoria is famous for its localized acid brines (e.g.., Dickson and Giblin, 2009; Long et al., 1992, 2009). Thiry et al. (2006) interpreted acidic groundwater alteration in south-central Australia to have produced weathering profiles rich in kaolinite, gypsum, alunite, and opal. Even some marginal marine environments have moderately acid (pH ~5) waters (i.e., Sammut et al., 1996). Southern Western Australia, and in particular the Yilgarn Craton, may simply be more advanced in acid brine evolution than the remainder of the continent. A combination of Australia’s old cratonic rocks, relatively low tectonic activity, relatively few carbonate rocks, and long wet-to-dry climate trend, as proposed by Long and Lyons (1990, 1992) over the past tens of millions of years, has likely resulted in the acid brine development for much of the continent.
Future of Acid Brines in Response to Global Climate Change and Human Land Use
In the twentieth century, both agriculture and mining had local influence on acid brine groundwater. A government-sponsored effort to turn the semi-arid eucalypt forests of inland southern Western Australia to crop and ranchland promoted the deforestation of the “wheat belt” region. With fewer trees to soak up the acid saline groundwater, the water table rose. Ranchers realized that cattle and sheep did not thrive with acid brines. Farmers found the only successful crops were grown high above the water table and irrigated with desalinized seawater piped a distance of hundreds of kilometers. Mining efforts have also used desalinized seawater pipelines. Both mining and agriculture import fresher water to the groundwater system and may be responsible for changing the volume of groundwater slightly, as well as potentially causing dissolution of some subsurface halite and other chemical sediments, and, perhaps in turn, increasing groundwater salinity. The limited volume of groundwater, in combination with its acidity, salinity, and high concentrations of some metals, make southern Western Australia a difficult place for human habitation.
Predictions for future climate suggest continued aridification in Australia due to global warming. Frederiksen et al. (2009) noted decreasing peak jet stream winds at ~30°S over southern Australia and predicted that this would cause increased temperatures, decreased autumn and winter rainfall, and prolonged droughts. Future aridification would continue a documented drying as evidenced by termination of perennial fluvial systems and shrinking of large saline lakes in Australia during the late Tertiary and Quaternary (Cohen et al., 2011; Salama, 1994; Van de Graaf et al., 1978; Zheng et al., 1998). This predicted drier scenario for the Yilgarn Craton suggests that ephemeral acid brine lakes will exist in a desiccated stage more often than in a flooded or evapo-concentrated stages. In addition, acid brine water tables may be lower in the future. More evaporation would result in lower pH and higher salinity. Therefore, the acid brine system across the Yilgarn may become more extreme but less voluminous.
Implications for Interpretations of Ancient Acid Brines in the Rock Record
Extreme acid brine environments similar to those in southern Western Australia have been recognized on Earth and Mars (e.g., Benison and Bowen, 2006; Benison et al., 1998; Kraus, 1998). In particular, some mid-Permian continental environments hosted extremely acid saline lakes and groundwaters that deposited redbeds and evaporites (Benison et al., 1998). The temporal and geographic extent of these Permian acid brine settings, and their relationship to Permian climate change and the end Permian mass extinction, are open scientific questions. Understanding the origin, evolution, and maintenance of modern natural acid brine environments may lead to more informed paleoenvironmental, paleoclimatic, and paleobiological interpretations about ancient acid brines.
Acknowledgments
Partial funding was provided by National Science Foundation grants EAR-0433040 and EAR-0719822 to K.C. Benison and EAR-0719892 to B.B. Bowen. Acknowledgment is made to the donors of the American Chemical Society–Petroleum Research Fund for partial support of this research. We thank C. Botero Sanchez, M.C. Hein, B.-y. Hong, E.A. Jagniecki, J.P. Knapp, D.A. LaClair, M. Mormile, F.E. Oboh-Ikuenobe, and S. Story for assistance in the field. We are indebted to Wayne Hitchcock and Stephen Wyche for logistical help and to Dave Long, Tim Lowenstein, and David Gray for discussions about acid brines. We thank Steven Whitmeyer for editorial handling and David Gray and an anonymous reviewer for constructive comments.
REFERENCES CITED
- Alpers, C.N., Rye, R.O., Nordstrom, D.K., White, L.D., and King, L., 1992, Chemical, crystallographic and stable isotopic properties of alunite and jarosite from acid-hypersaline Australian lakes: Chemical Geology, v. 96, p. 203–226, doi: 10.1016/0009-2541(92)90129-S.
- Anand, R.R., and Paine, M., 2002, Regolith geology of the Yilgarn Craton: Australian Journal of Earth Sciences, v. 49, p. 3–162, doi: 10.1046/j.1440-0952.2002.00912.x.
- Benison, K.C., 2008, Life and death around acid-saline lakes: Palaios, v. 23, p. 571–573, doi: 10.2110/palo.2008.S05.
- Benison, K.C., 2012, Extremely acid saline environments as biospheres: Approaching the limits of life: Abstracts, 112th American Society for Microbiology Annual Meeting, San Francisco, California, USA, p. 92.
- Benison, K.C., and Bowen, B.B., 2006, Acid saline lake systems give clues about past environments and the search for life on Mars: Icarus, v. 183, p. 225–229, doi: 10.1016/j.icarus.2006.02.018.
- Benison, K.C., and Bowen, B.B., 2013, Extreme sulfur-cycling in acid brine lake environments of Western Australia: Chemical Geology, v. 351, p. 154–167, doi: 10.1016/j.chemgeo.2013.05.018.
- Benison, K.C., Goldstein, R.H., Wopenka, B., Burruss, R.C., and Pasteris, J.D., 1998, Extremely acid Permian lakes and ground waters in North America: Nature, v. 392, p. 911–914, doi: 10.1038/31917.
- Benison, K.C., Bowen, B.B., Oboh-Ikuenobe, F.E., Jagniecki, E.A., LaClair, D.A., Story, S.L., Mormile, M.R., and Hong, B.-y., 2007, Sedimentology of acid saline lakes in southern Western Australia: Newly described processes and products of an extreme environment: Journal of Sedimentary Research, v. 77, no. 5, p. 366–388, doi: 10.2110/jsr.2007.038.
- Benison, K.C., Jagniecki, E.A., Edwards, T.B., Mormile, M.C., and Storrie-Lombardi, M.C., 2008, “Hairy blobs”: Microbial suspects from modern and ancient ephemeral acid saline evaporites: Astrobiology, v. 8, no. 4, p. 807–821, doi: 10.1089/ast.2006.0034.
- Bowen, B.B., and Benison, K.C., 2009, Geochemical characteristics of naturally acid and alkaline saline lakes in southern Western Australia: Applied Geochemistry, v. 24, p. 268–284, doi: 10.1016/j.apgeochem.2008.11.013.
- Bowen, B.B., Benison, K.C., and Story, S., 2012, Early diagenesis by modern acid brines in Western Australia and implications for the history of sedimentary modification on Mars, in Grotzinger, J., and Milliken, R., eds., Sedimentary Geology of Mars: SEPM Special Publication 102, p. 229–252.
- Bowen, B.B., Story, S., Oboh-Ikuenobe, F.E., and Benison, K.C., 2013, Differences in regolith weathering history at an acid and neutral saline lake on the Archean Yilgarn Craton and implications for acid brine evolution: Chemical Geology, v. 356, p. 126–140, doi: 10.1016/j.chemgeo.2013.08.005.
- Clarke, J.D.A., 1993, Stratigraphy of the Lefroy and Cowan palaeodrainages, Western Australia: Journal of the Royal Society of Western Australia, v. 76, p. 13–23.
- Clarke, J.D.A., 1994, Evolution of the Lefroy and Cowan palaeodrainage channels, Western Australia: Australian Journal of Earth Sciences, v. 41, p. 55–68, doi: 10.1080/08120099408728113.
- Clarke, J.D.A., Bone, Y., and James, N.P., 1996, Cool-water carbonates in an Eocene paleoestuary, Norseman Formation, Western Australia: Sedimentary Geology, v. 101, p. 213–226, doi: 10.1016/0037-0738(95)00066-6.
- Cohen, T.J., Nanson, G.C., Jansen, J.D., Jones, B.G., Jacobs, Z., Treble, P., Price, D.M., May, J.-H., Smith, A.M., Ayliffe, L.K., and Hellstrom, J.C., 2011, Continental aridification and the vanishing of Australia’s megalakes: Geology, v. 39, p. 167–170, doi: 10.1130/G31518.1.
- Conner, A.J., and Benison, K.C., 2013, Acidophilic halophilic microorganisms in fluid inclusions in halite from Lake Magic, Western Australia: Astrobiology, v. 13, no. 9, p. 850–860, doi: 10.1089/ast.2012.0956.
- Dickson, B.L., and Giblin, A.M., 2009, Features of acid-saline systems of Southern Australia: Applied Geochemistry, v. 24, p. 297–302, doi: 10.1016/j.apgeochem.2008.11.011.
- Eugster, H.P., 1970, Chemistry and origin of the brines of Lake Magadi, Kenya, in Morgan, B.A., Jones, B.F., Kullerud, G., Osborn, E.F., and Holser, W.T., eds., Fiftieth Anniversary Symposia: Mineralogy and Petrology of the Upper Mantle Sulfides, Mineralogy and Geochemistry of Non-Marine Evaporites: Mineralogical Society of America Special Paper 3, p. 213–235.
- Eugster, H.P., 1980, Geochemistry of evaporitic lacustrine deposits: Annual Review of Earth and Planetary Sciences, v. 8, p. 35–63, doi: 10.1146/annurev.ea.08.050180.000343.
- Eugster, H.P., and Hardie, L.A., 1978, Saline lakes, in Lerman, A., ed., Lakes: Chemistry, Geology, Physics: New York, Springer-Verlag, p. 237–289.
- Eugster, H.P., and Jones, B.J., 1979, Behavior of major solutes during closed-basin brine evolution: American Journal of Science, v. 279, p. 609–631, doi: 10.2475/ajs.279.6.609.
- Foster, R.M., and Benison, K.C., 2006, Acid saline weathering experiments using acid lake host rocks from Western Australia: Preliminary results: Geological Society of America Abstracts with Programs, v. 38, no. 7, p. 502.
- Frederiksen, J.S., Frederiksen, C.S., and Osbrough, S.L., 2009, Modelling of changes in Southern Hemisphere weather systems during the 20th century: 18th World IMACS/MODSIM Congress, Cairns, Australia, p. 2562–2568.
- Gray, D.J., 2001, Hydrogeochemistry in the Yilgarn Craton: Geochemistry Exploration Environment Analysis, v. 1, p. 253–264, doi: 10.1144/geochem.1.3.253.
- Gregory, J.W., 1914, The lake system of Westralia: The Geographical Journal, v. 43, p. 656–664, doi: 10.2307/1779150.
- Hardie, L.A., and Eugster, H.P., 1970, The evolution of closed-basin brines, in Morgan, B.A., Jones, B.F., Kullerud, G., Osborn, E.F., and Holser, W.T., Fiftieth Anniversary Symposia: Mineralogy and Petrology of the Upper Mantle Sulfides, Mineralogy and Geochemistry of Non-Marine Evaporites: Mineralogical Society of America Special Paper 3, p. 273–290.
- Hingston, F.J., and Gailitis, V., 1976, The geographic variation of salt precipitated over Western Australia: Australian Journal of Soil Research, v. 14, p. 319–335, doi: 10.1071/SR9760319.
- Kraus, M.J., 1998, Development of potential acid sulfate paleosols in Paleocene floodplains, Bighorn Basin, Wyoming, U.S.A.: Palaeogeography, Palaeoclimatology, Palaeoecology, v. 144, p. 203–224, doi: 10.1016/S0031-0182(98)00086-8.
- Langworthy, T.A., 1978, Microbial life in extreme pH values, in Kushner, D.J., ed., Microbial Life in Extreme Environments: London, Academic Press, p. 279–315.
- Lawrance, L.M., 2001, Multi-element dispersion within salt-lake environments: Case study of the buried Hannan South gold deposit, Western Australia: Geochemistry Exploration Environment Analysis, v. 1, p. 323–339, doi: 10.1144/geochem.1.4.323.
- Long, D.T., and Lyons, W.B., 1990, Aridity, continental weathering, and groundwater chemistry: Geological Society of America Abstracts with Programs, v. 22, no. 6, p. 295.
- Long, D.T., and Lyons, W.B., 1992, Aridity, continental weathering, and groundwater chemistry: GSA Today, v. 2, no. 9, p. 185–190.
- Long, D.T., Fegan, N.E., Lyons, W.B., Hines, M.E., Macumber, P.G., and Giblin, A., 1992, A geochemistry of acid-brines: Lake Tyrrell, Australia: Chemical Geology, v. 96, p. 33–52, doi: 10.1016/0009-2541(92)90120-T.
- Long, D.T., Lyons, W.B., and Hines, M.E., 2009, Influence of hydrogeology, microbiology, and landscape history of acid hypersaline waters, N.W. Victoria: Applied Geochemistry, v. 24, p. 285–296, doi: 10.1016/j.apgeochem.2008.11.012.
- Lowenstein, T.K., and Hardie, L.A., 1985, Criteria for the recognition of salt-pan evaporites: Sedimentology, v. 32, p. 627–644, doi: 10.1111/j.1365-3091.1985.tb00478.x.
- Lowenstein, T.K., Spencer, R.J., and Pengxi, Z., 1989, Origin of ancient potash evaporates: Clues from the modern nonmarine Qaidam Basin of Western China: Science, v. 245, p. 1090–1092, doi: 10.1126/science.245.4922.1090.
- Mann, A.W., 1983, Hydrogeochemistry and weathering on the Yilgarn Block, Western Australia—Ferrolysis and heavy metals in continental brines: Geochimica et Cosmochimica Acta, v. 47, p. 181–190, doi: 10.1016/0016-7037(83)90131-X.
- McArthur, J.M., Turner, J., Lyons, W.B., and Thirlwall, M.F., 1989, Salt sources and water-rock interaction on the Yilgarn Block, Australia: Isotopic and major element tracers: Applied Geochemistry, v. 4, p. 79–92, doi: 10.1016/0883-2927(89)90060-7.
- McArthur, J.M., Turner, J., Lyons, W.B., Osborn, A.O., and Thirlwall, M.F., 1991, Hydrochemistry on the Yilgarn Block, Western Australia: Ferrolysis and mineralization in acidic brines: Geochimica et Cosmochimica Acta, v. 55, p. 1273–1288, doi: 10.1016/0016-7037(91)90306-P.
- Mormile, M.R., Hong, B.-y., and Benison, K.C., 2009, Molecular analysis of the microbial communities of Mars-analog lakes in Western Australia: Astrobiology, v. 9, p. 919–930, doi: 10.1089/ast.2008.0293.
- Oren, A., 2010, Acidophiles: New York, John Wiley & Sons, doi: 10.1002/9780470015902.a0000336.pub2.
- Salama, R., 1994, The evolution of saline lakes in the relict drainage of the Yilgarn River, Western Australia, in Renaut, R.W., and Last, W.M., eds., Sedimentology and Geochemistry of Modern and Ancient Saline Lakes: SEPM Special Publication 50, p. 187–199.
- Sammut, J., White, I., and Melville, M.D., 1996, Acidification of an estuarine tributary in eastern Australia due to drainage of acid sulphate soils: Marine
- & Freshwater Research, v. 47, p. 669–684, doi: 10.1071/MF9960669.
- Spencer, R.J., 2000, Sulfate minerals in evaporite deposits, in Alpers, C.N., Jambor, J.L., and Nordstrom, K., eds., Sulfate Minerals: Crystallography, Geochemistry, and Environmental Significance: Reviews in Mineralogy and Geochemistry, v. 40, p. 173–192.
- Story, S., Bowen, B.B., Benison, K.C., and Schulze, D.G., 2010, Authigenic phyllosilicates in modern acid saline lake sediments and implications for Mars: Journal of Geophysical Research Planets, v. 115, E12012, 17 p., doi: 10.1029/2010JE003687.
- Thiry, M., Milnes, A.P., Ravot, V., and Simon-Coincon, R., 2006, Interpretation of paleoweathering features and successive silicifications in Tertiary regolith of inland Australia: Journal of the Geological Society, v. 163, p. 723–736, doi: 10.1144/0014-764905-020.
- Van de Graaf, W.J.E., Crowe Bunting, J.A., and Jackson, M.J., 1978, Relict early Cainozoic drainages in arid Western Australia: Annals of Geomorphology, N.F., v. 21, p. 379–400.
- Zheng, H., Wyrwoll, K.-H., Li, Z., and Powell, C.M., 1998, Onset of aridity in southern Western Australia—A preliminary paleomagnetic appraisal: Global and Planetary Change, v. 18, p. 175–187, doi: 10.1016/S0921-8181(98)00019-8.

 Figure 1
Figure 1 Figure 2
Figure 2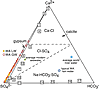 Figure 3
Figure 3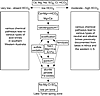 Figure 4
Figure 4 Figure 5
Figure 5