Full Text View
Volume 24 Issue 8 (August 2014)
GSA Today
Article, pp. 4-10 | Abstract | PDF (3.1MB)
The heavy metal contamination of Lake Junín National Reserve, Peru: An unintended consequence of the juxtaposition of hydroelectricity and mining
| Table of Contents |
|---|
|
Search GoogleScholar for
Search GSA Today |
| View past Presidential Addresses. |
ABSTRACT
Hydraulic engineering is increasingly relied upon to provide the necessary dry-season discharge for Peru’s hydroelectricity generation. Redirecting stream flow can yield unintended consequences, however, and here we document the wholesale contamination of the Lake Junín National Reserve by acid mine drainage from the Cerro de Pasco mining district. Since construction of the Upamayo Dam in 1932, the Río (river) San Juan, which drains the Cerro de Pasco region, has been seasonally redirected into Lake Junín. As a result, the upper several decimeters of sediment in the lake contain peak concentrations of Cu, Zn, and Pb of ~6000 ppm, ~50,000 ppm, and ~2000 ppm, respectively, with the latter two greatly exceeding the United States Environmental Protection Agency (EPA) limits for the entire 150 km2 lake basin. That the source of the contamination to Lake Junín is acid mine drainage from Cerro de Pasco is supported by spatial gradients in metal concentrations, authigenic calcite (marl) concentrations, and the isotopic record of Junín water. Today, the upper 50 cm of sediment in Lake Junín contain ~60,400, 897,600, and 40,900 metric tons of Cu, Zn, and Pb, respectively, which is equivalent to ~5.1 years’ worth of Zn extraction and ~0.7 years’ worth of Pb extraction from mining operations at Cerro de Pasco at current rates.
*Email:
DOI: 10.1130/GSATG200A.1
Manuscript received 23 Sept. 2013; accepted 18 Dec. 2013.
Introduction
About 60% of Peru’s electricity is generated by hydropower (World Bank, 2013), which during the dry season relies heavily on glacial meltwater to augment stream flow. During the austral winter months (June, July, and August [JJA]), precipitation in the high Andes is <5% of the annual total, and it has been estimated for one drainage basin in north-central Peru that during these months ~40% of river discharge comes from glacial meltwater (Mark et al., 2005). The ongoing reduction in ice cover in Peru that began early in the twentieth century has reduced the aerial extent of glacial ice in some areas by ~30% (Vuille et al., 2008). Climate models project that warming will be pronounced in the highest elevation regions of the tropical Andes (Bradley et al., 2006), and thus acceleration in ice loss is likely. In order to maintain dry season river discharge and energy generation for a growing Peruvian population, the hydropower industry in Peru has turned to hydraulic engineering, including dam construction, to ensure river discharge and hydroelectric production. This study highlights an unintended consequence of early dam construction in the Cerro de Pasco region of the central Peruvian Andes, a region that has been a focal point of Peruvian mining operations for centuries.
When Francisco Pizarro conquered the Incan Empire in 1533, he found a longstanding legacy of metallurgy and mining activity spanning almost a millennium (Abbott and Wolfe, 2003). Pre-colonial mining occurred in Cerro de Pasco, with the earliest evidence for anthropogenic lead enrichment by aerosolic fallout in nearby lakes at ca. 600 CE (Cooke et al., 2009). The Cerro de Pasco mining district is among the most extensively worked mining districts in Peru, and during the last five years of the eighteenth century, silver output in Cerro de Pasco surpassed even that of Potosí, Bolivia (Hunefeldt, 2004).
The Peruvian War of Independence (1809–1824) temporarily crippled the silver industry at Cerro de Pasco, and the final battles for independence took place among the silver mines themselves. In the first two decades after independence, Cerro de Pasco produced 65% of Peruvian silver, and to support the mining industry, a central railway was constructed between Lima and La Oroya in the late nineteenth century (Klarén, 2000), which was later extended to Cerro de Pasco. The railroad also allowed for the transition to copper production in 1897 (Becker, 1983). An American engineer, A. William McCune, explored the Peruvian cordillera searching for copper, and he found plenty of it among the exhausted silver ores of Cerro de Pasco (Becker, 1983). McCune helped organize a syndicate (1900–1901) that included J.P. Morgan to finance the Peruvian copper venture. Named the Cerro de Pasco Investment Corporation, and later the Cerro de Pasco Copper Corporation, the company constructed the first copper smelter in 1906. The volume of ore production at Cerro de Pasco soon justified construction of a large central smelter, completed in 1922, and by 1931 the Cerro smelter held monopoly over the refining of all nonferrous metals in Peru (Becker, 1983).
In order to generate hydroelectricity for Cerro de Pasco’s operations, the Upamayo Dam was constructed in 1932 (Shoobridge, 2006). The Upamayo Dam is located in the uppermost reach of the Río Mantaro, immediately downstream of the confluence between the Río San Juan, which drains southward from Cerro de Pasco, and the outflow of Lake Junín, the largest lake entirely within Peru (Fig. 1). Cerro’s operations grew to include lead and zinc by 1952 (Klarén, 2000), but political turmoil disrupted production during the second half of the twentieth century, when in 1968 a bloodless coup d’état against President Belaúnde led to rule by a military junta that lasted until 1975. In 1971, the Cerro de Pasco Copper Corporation was nationalized under the name Centromín (Klarén, 2000). Deregulation in the 1990s allowed for the reacquisition of the mining operations at Cerro de Pasco by the Peruvian based company Compañía Minera Volcán S.A. (Gurmendi, 2006).
The location of the Upamayo Dam and the small reservoir upstream from it has resulted in the discharge of Río San Juan waters, once destined for the Río Mantaro, directly into Lake Junín. This redirecting of the Río San Juan into Lake Junín began in 1932 and is most prevalent during the dry season (JJA) when the level of Lake Junín typically drops by ~2 m (Pedersen et al., 1999). This paper documents the impact of acid mine drainage from Cerro de Pasco into Lake Junín, which in 1974 was designated a Peruvian National Wildlife Reserve.
Methodology
Six sediment cores (0.6–1.3 m long) were acquired with a Verschuren surface corer (Verschuren, 1993) between 2002 and 2008 from various locations in Lake Junín (Fig. 1). Results from the 2002 core are reported by Veliz (2001). Surface sediment and water samples were acquired in 2013 from three locations along the Río San Juan between Cerro de Pasco and the Upamayo Dam. Sediment cores were subsampled in the field at 0.5- and 1.0-cm increments and transported to the sediment core laboratory at Union College (Schenectady, New York, USA) for analysis of exchangeable (adsorbed) metals. All sediment samples were freeze-dried and disaggregated in an agate mortar and pestle, and between 50 and 100 mg of sample was then placed in 14 ml FalconTM test tubes. To each sample we added 9.5 ml of milliQ deionized water and 1.0 ml high-purity (70%) HNO3. Samples were then shaken horizontally for 12 h, refrigerated vertically for 24 h, and returned to room temperature for 1 h. Subsequently, 1.0 ml of sediment-free supernatant was extracted and diluted with 9 ml of milliQ deionized water; these samples were analyzed for Mn, Fe, Co, Cu, Zn, Sr, Ba, and Pb by a Perkin Elmer Sciex ELAN 6100 DRC inductively coupled plasma–mass spectrometer (ICP-MS). A blank solution was prepared with each batch of samples, and blanks and standards were analyzed by ICP-MS at regular intervals throughout sample runs, which ranged from ~50–150 samples. Here we focus on Cu, Zn, and Pb; concentration data for all metals are available in the GSA Supplemental Data Repository 1 (Table S1).
1GSA supplemental data item 2014200—concentration data for metals, total carbon, total CaCO3, total organic carbon, oxygen and carbon isotope data, and age model data for each core—is online at www.geosociety.org/pubs/ft2014.htm. You can also request a copy from GSA Today, P.O. Box 9140, Boulder, CO 80301-9140, USA; .
We measured weight percentage total carbon (TC) and weight percentage total inorganic carbon (TIC) by coulometry. For the measurement of TC, we combusted samples at 1000 °C using a UIC 5200 automated furnace and analyzed the resultant CO2 by coulometry using a UIC 5014 coulometer. Similarly, we measured TIC by acidifying samples using a UIC 5240 acidification module and measured the resultant CO2 by coulometry. We calculated weight percentage total organic carbon (TOC) from TOC = TC − TIC; weight percentage TIC was converted to % calcite based on stoichiometry. All coulometry data are available in the GSA Supplemental Data Repository (Table S2; see footnote 1).
Age models (Fig. 2 and Table S3 [see footnote 1]) for sediment cores were developed from a combination of radiocarbon dates on plant macrofossils and correlation of the significant rise in anthropogenic Pb to the 210Pb-dated record of aerosolic Pb deposition since 1625 CE in regional glacial lakes within ~25 km of Lake Junín (Cooke et al., 2009; Bird, 2009). In addition, we correlated the 18O record of marl in one Junín core (Core B, Fig. 1) with the 210Pb-dated 18O record of marl in nearby Laguna Pumacocha (Fig. 1) (Bird et al., 2011a).
|
Location of Lake Junín in the central Peruvian Andes. Red dots in Lake Junín are short-core locations; red dots outside of Lake Junín drainage basin denote the location of lakes with published records of aerosolic metal contamination (Cooke et al., 2009; Bird et al., 2011a); purple dots along Río San Juan mark the location of modern alluvium collected in July 2013. |
|
Age-depth models for sediment cores used in this study. |
We determined the history and source of metal deposition in Lake Junín by comparing sediment cores from within Lake Junín with those from nearby lakes that are not connected hydrologically to Junín. The downcore variation in metal concentration is the simplest means of comparison, but because the concentration of any particular metal is inversely affected by the deposition rate of all other metals and non-metals (e.g., clastic sediment, organic matter, and marl), a more rigorous metric is to calculate the deposition rate, or flux, of each metal analyzed. Flux is the product of dry sediment bulk density (mg cm−3), sedimentation rate (cm yr−1), and the concentration of a particular metal (ppm). The units of flux are thus mass of metal X per unit area of lake floor per year (e.g., µg cm−2 yr−1), and the flux of any component to a lake is independent of the changing rates of deposition of all other components.
Data and Discussion
Downcore Trends
There are consistent downcore trends in metal concentrations in surface cores throughout Lake Junín. Baseline (pre–twentieth century) concentrations of all metals are low, and concentrations rise abruptly in the upper several decimeters of all cores. For example, background levels of Cu, Zn, and Pb in Core B are <1 ppm, <10 ppm, and <1 ppm, respectively (Fig. 3). Concentrations of these metals rise abruptly in the early twentieth century to >450 ppm, >30,000 ppm, and >150 ppm, respectively. In cores acquired near the northern end of Lake Junín (2008 Cores C and G), peak concentrations of Cu, Zn, and Pb reach ~6000 ppm, ~50,000 ppm, and ~2000 ppm, respectively. These concentrations are similar to those measured on surface sediments in Lake Junín by Pedersen et al. (1999). Our best dated core (2008 Core B) reveals that metal concentrations began to increase as early as ca. 400 CE, consistent with evidence of pre-Incan smelting by the Tiwanaku and Wari Empires on the Altiplano of southern Peru and Bolivia (Cooke and Abbott, 2008). However, metal concentrations in Lake Junín during this time are ~1/100th of the concentration of metals in sediments deposited during the twentieth century.
|
Downcore variation in Cu, Zn, Pb, calcite, and 18O and 13C of marl in Lake Junín short core B (Fig. 1). Smoothed record of 18O of marl in Laguna Pumacocha (Fig. 1) (Bird et al., 2011a) reveals isotopic composition of regional precipitation; simultaneous isotopic depletion of Lake Junín and increase in metal concentrations records the influx of isotopically light acid mine drainage from the Cerro de Pasco region via the Río San Juan due to construction of the Upamayo Dam ca. 1932 CE. VPDB—Vienna Pee Dee Belemnite. |
The concentration of authigenic calcite (marl) in Junín sediments declines abruptly when metal concentrations increase (Fig. 3). For much of the past 12,000 years, Lake Junín has been generating calcite at ~0.85 mm yr−1 (Seltzer et al., 2000), and calcite represents ~80%–100% of the mass of Holocene sediment until the twentieth century. This calcite is produced both organically as mollusk shells and ostracode carapaces and inorganically as a precipitate on the leaves of the submerged macrophyte Chara. The abrupt decline in calcite concentration may record the acidification of Lake Junín water by acid mine drainage to the point that HCO3− and Ca+ remain soluble and no longer precipitate calcite. Alternatively, the inverse relationship between organic carbon (from algal remains and plant macrofossils) and calcite concentration (Fig. 3) may indicate that lake eutrophication resulted in the post-depositional dissolution of authigenic calcite in the lake sediments. This latter scenario is apparently responsible for the decline in CaCO3 concentrations in Minnesota lakes; there, Dean (1999) concluded that organic carbon concentrations in excess of 12% generate enough carbonic acid in sediment pore water to dissolve all contemporaneously deposited marl. Because the pH of Lake Junín (7.7–8.5; Flusche et al., 2005) is not anomalously low for calcite precipitation, it is possible that lake eutrophication was the cause of the abrupt decline in CaCO3 concentrations. Of course, some combination of acid mine drainage and lake eutrophication is possible, and an influx of nutrients may have occurred simultaneously with the introduction of heavy metals into the lake.
A comparison between the 18O record from Junín marl and that from nearby Laguna Pumacocha confirms that the introduction of heavy metals to Lake Junín was associated with a significant input of river water (Fig. 3). The 18O record from both lakes shows that they generally track one another over the Holocene, reflecting regional changes in the isotopic composition of precipitation (Vuille et al., 2012). However, beginning ca. 1920 CE, the Pumacocha record reveals a positive shift of 1.0‰ that is consistent with other regional isotopic records from ice cores and speleothems (Bird et al., 2011b), whereas the Junín record shifts abruptly 2‰ in the negative direction (Fig. 3). This discrepancy can be explained by an increase in the seasonal discharge of the Río San Juan directly into Lake Junín as a result of the construction of the Upamayo Dam (Fig. 1) in 1932 CE. The introduction of isotopically 18O-depleted river water into evaporatively 18O-enriched Lake Junín would result in a lake-wide isotopic depletion of marl. Further, an abrupt decrease from ~11–5‰ in the 13C of marl occurred at the same time as the negative shift in oxygen values (Fig. 3). This may also reflect the influx of Río Santa water into Lake Junín, which for much of the Holocene had been accumulating authigenic calcite with 13C values that are highly enriched (between 8 and 14‰) as a result of degassing (Seltzer et al., 2000). Increased Río Santa inflow may also have contributed isotopically light carbon from flooded soils and wetlands into Lake Junín.
Spatial Trends
The spatial distribution of heavy metals in Lake Junín sediments further confirms that the source of these contaminants is indeed the Río San Juan (Fig. 4). Although San Juan discharge may well have seasonally entered Lake Junín prior to the construction of the Upamayo Dam, construction of the dam increased this discharge substantially. The average concentration of Cu and Pb from ca. 1850 CE to present decreases markedly with increasing distance from the confluence of the Río San Juan with Lake Junín (Fig. 4). In the most distal (southerly) cores, the concentration of these metals approaches that of Cu and Pb in the three nearby lakes that are not hydrologically linked to Cerro de Pasco or any other mining district (Fig. 1; Cooke et al., 2009; Bird et al., 2011a). Metal concentrations in these latter lakes, therefore, provide a record of regional background deposition by aerosolic input only, and this, in turn, confirms the point-source origin of much of the metal contamination to Lake Junín. The concentration of Zn does not show a similar decline with increasing distance from the Río San Juan (Fig. 4), and this may reflect a complex process of Zn recycling between sediment pore waters and lake water, as discussed by Pedersen et al. (1999). Pedersen et al. report Zn concentrations in interfacial pore waters in Lake Junin that are an order of magnitude higher than Zn concentrations both in the superjacent water column and in subjacent pore waters 10 cm below the sediment-water interface. Apparently, Zn can be recycled from the sediment to the water column by oxidation of labile organic compounds at the sediment-water interface (Pedersen et al., 1999). Presumably, this recycled Zn may then be resorbed onto organic compounds that can be deposited further into the lake basin, and this may explain the increasing trend in Zn concentration with increasing distance from the confluence of the Río San Juan.
|
Variation in Cu, Zn, and Pb in Lake Junín with increasing distance from Cerro de Pasco plotted as both mean concentration (ppm) and mean flux (µg cm−2 yr−1) for the period 1850 CE to present. Distance is radial distance and does not reflect the true thalweg distance of Río San Juan. Río San Juan enters Lake Junín ~25 km south of Cerro de Pasco (Fig. 1). Also plotted are the mean concentration and flux of Cu, Zn, and Pb to three lakes (Lagunas Chipian, Pumacocha, and Llamacocha; Fig. 1) (Cooke et al., 2009; Bird et al., 2011a) that are not hydrologically linked to any mining districts, and, thus, these lakes can only receive aerosolic inputs of metals. The concentrations of Cu, Zn, and Pb for three samples of modern alluvium collected from the channel of the Río San Juan and four samples of modern river and lake water are plotted for comparison. Metal concentration limits for sediments from the U.S. Environmental Protection Agency (U.S. EPA, 1993) are plotted for Zn and Pb; those for Cu are 4300 ppm and are off scale. |
In general, the average flux of Cu, Zn, and Pb reveals lower input rates of these metals with increasing distance from the confluence of the Río San Juan (Fig. 4). The decline in Zn flux with increasing distance from the Río San Juan confluence seems to be at odds with the lack of any similar trend in Zn concentration. It is possible that reduced Zn flux with increasing distance into Lake Junín may be compensated for by a reduction in the deposition rate of other components of the lake sediment, thus allowing for Zn concentration to remain high in the more distal samples. That Cu and Pb do not also show this trend may stem from their very high concentrations in proximal samples, which are 2–4 times as high as samples from even moderately distal sites (Fig. 4). Pedersen et al. (1999) estimated a diffusive efflux rate for Zn from surface sediments into the overlying water column of ~25 µg cm−2 yr−1, which is of the same order of magnitude as the average flux of Zn since 1850 CE for the more distal sites in Lake Junín. Recycled Zn, therefore, may be the major source of Zn to distal sites.
Magnitude of Contamination
Comparison between the average Cu, Zn, and Pb concentration in sediment deposited since 1850 CE with limits set for sediments by the EPA (U.S. EPA, 1993) reveals the relative magnitude of the contaminant threat posed by these metals (Fig. 4). Whereas only peak concentrations of Cu in the most proximal cores (Cores G and C, Fig. 1) exceed the EPA limit of 4300 ppm, and mean Cu concentrations (1850 CE to present) are considerably lower than this level, average concentrations of Zn and Pb exceed EPA limits for these metals of 7500 and 420 ppm, respectively, nearly everywhere in Lake Junín. Peak concentrations of Zn and Pb within proximal cores in Lake Junín exceed EPA limits by one order of magnitude, and the peak in Zn concentration within cores (~50,000 ppm) is nearly uniform throughout the lake basin.
Comparison between the concentration of Cu, Zn, and Pb in the modern alluvium of the Río San Juan between Cerro de Pasco and Lake Junín (Fig. 1) and the concentration of these elements in proximal cores reveals that some progress may have been made in reducing the output of Zn from tailings piles and tailings ponds (Fig. 4). The concentration of Zn in modern alluvium is much lower than the average Zn concentration in Junín sediment deposited since 1850 CE anywhere in the lake. The concentrations of Cu and Pb, however, show mixed results, and some samples of modern alluvium are as concentrated in these elements as the most contaminated sediment in Lake Junín. Pedersen et al. (1999) and Martin et al. (2001) noted that alluvium ponded on the upstream side of the Upamayo Dam is subjected to seasonal exposure and inundation. During the dry season, when these sediments are exposed and unsaturated, sulfide oxidation and the resultant acidification of pore waters occurs; these workers report pore water pHs as low as 3.2 in this location. These acidic conditions remobilize Cu, Zn, and Pb from both sulfide minerals and secondary oxidation products (Pedersen et al., 1999), and the redissolved metals are then flushed into Lake Junín during the subsequent wet season when rising lake levels inundate these deposits. Thus, the alluvium derived from the mine tailings of Cerro de Pasco and ponded behind the Upamayo Dam serves as a long-term source of metal contamination to Lake Junín. The pumping of metals from these deposits into Lake Junín would be dramatically reduced if these deposits were permanently submerged with anoxic lake water (Martin et al., 2001).
To fully appreciate the scale of the contamination of Lake Junín that has resulted from decades of uncontrolled acid mine drainage from Cerro de Pasco, we consider the total mass of Cu, Zn, and Pb in the upper 50 cm of lake sediments (Table 1). We calculate the average concentration of these metals in the upper 50 cm of our six sediment cores and take this to be an approximation of the average concentration of Cu, Pb, and Zn for the upper 50 cm of the lake basin. The resultant mass of Cu, Zn, and Pb in Lake Junín’s uppermost 50 cm is 60,425, 897,588, and 40,900 metric tons, respectively. The average annual production of Zn and Pb from 2009 to 2011 from the mining operations at Cerro de Pasco are 177,000 and 53,333 metric tons, respectively (Volcan, 2013) (Table 1). Thus, at current rates of extraction, there are 5.1 years’ worth of Zn and 0.7 years’ worth of Pb stored in the upper 50 cm of Lake Junín.
|
Mass of Cu, Zn, and Pb in upper 50 cm of sediment in Lake Junín relative to annual output from mining activities in Cerro de Pasco. |
The concentration of Cu, Zn, and Pb in water samples from the Río San Juan and Lake Junín are lower than sediment samples from the same sampling locations (Fig. 4). Concentrations of Cu, Zn, and Pb in water samples range from 111 to 282 ppm, 123 to 1603 ppm, and 3 to 18 ppm, respectively, and all exceed maximum contaminant levels (MCLs) set by the U.S. EPA (EPA, 2009) and Peru’s Permissible Maximum Limits (LMPs; Ministerio del Ambiente, 2010) for effluent discharge from mining activities by ~2 orders of magnitude or more. The aforementioned recycling of Zn from lake bottom sediments into the water column contributes a significant source of dissolved Zn in Lake Junín (Pedersen et al., 1999). It would seem, then, that until a concerted effort focuses on preventing the remobilization of metals from the ponded sediment behind the Upamayo Dam and a sufficient thickness of uncontaminated sediment buries the heavily contaminated sediments of Lake Junín, the influx of metals to Junín waters will continue, and concentrations will exceed MCLs and LMPs for the foreseeable future.
Conclusions
By virtue of the construction and location of the Upamayo Dam, much of the history of mining at Cerro de Pasco has been unwittingly recorded in the sediments of Lake Junín. Though Lake Junín and its surrounding wetlands were designated as a national reserve in 1974 to protect the rich avian life that the lake supports, decades of mine runoff from Cerro de Pasco have made the sediments of Lake Junín among the most polluted in Peru. The Upamayo Dam, which was constructed for hydroelectricity generation, is not the source of these contaminants, and were it not for the dam, the bulk of the metal pollution would have been destined for the Río Mantaro and the upper Amazon Basin. If this had been allowed to occur, the concentration of metals in the upper Amazon Basin would have been lower than that present in Lake Junín due to the dilution of metals over thousands of kilometers of river bottom. Among the biggest challenges that will face any attempt to mitigate the environmental disaster that has befallen Lake Junín are finding ways to stop the recycling of Zn from the lake bottom and the remobilization of all metals from the seasonally exposed and submerged deposits that are trapped behind the Upamayo Dam. As future hydraulic engineering projects are developed in Peru and elsewhere, it would behoove all not to repeat the mistakes that are recorded in the mud of Lake Junín.
Acknowledgments
This work was supported by the Faculty Research Fund of Union College and by National Science Foundation grants ATM-0502464 and EAR-1003711 to DTR. We are grateful to Matt Manon of the Union College Geology Department for analytical assistance and to two anonymous reviewers for their efforts to improve the manuscript.
REFERENCES CITED
- Abbott, M.B., and Wolfe, A., 2003, Intensive pre-Incan metallurgy recorded by lake sediments from the Bolivian Andes: Science, v. 301, p. 1893–1895, doi: 10.1126/science.1087806.
- Becker, D.G., 1983, The New Bourgeoisie and the Limits of Dependency: Mining, Class, and Power in “Revolutionary” Peru: Princeton, New Jersey, Princeton University Press, 419 p.
- Bird, B.W., 2009, Millennial- to annual- scale Holocene climate change in the Alaskan arctic and tropical Andes inferred from physical sedimentological and geochemical indicators preserved in finely laminated alpine lake sediment archives [Ph.D. thesis]: Pittsburgh, University of Pittsburgh, 117 p.
- Bird, B.W., Abbott, M.B., Vuille, M., Rodbell, D.T., Stansell, N.D., and Rosenmeier, M.F., 2011a, A 2,300-year-long annually resolved record of the South American summer monsoon from the Peruvian Andes: PNAS, v. 108, p. 8583–8588, doi: 10.1073/pnas.1003719108.
- Bird, B.W., Abbott, M.B., Rodbell, D.T., and Vuille, M., 2011b, Holocene tropical South American hydroclimate revealed from a decadally resolved lake sediment 18O record: Earth and Planetary Science Letters, v. 310, p. 192–202, doi: 10.1016/j.epsl.2011.08.040.
- Bradley, R.S., Vuille, M., Diaz, H.F., and Vergara, W., 2006, Threats to water supplies in the tropical Andes: Science, v. 312, p. 1755–1756, doi: 10.1126/science.1128087.
- Cooke, C.A., and Abbott, M.B., 2008, A paleolimnological perspective on industrial-era metal pollution in the central Andes, Peru: The Science of the Total Environment, v. 393, p. 262–272, doi: 10.1016/j.scitotenv.2007.12.034.
- Cooke, C.A., Wolfe, A.P., and Hobbs, W.O., 2009, Lake-sediment geochemistry reveals 1400 years of evolving extractive metallurgy at Cerro de Pasco, Peruvian Andes: Geology, v. 37, p. 1019–1022, doi: 10.1130/G30276A.1.
- Dean, W.E., 1999, The carbon cycle and biogeochemical dynamics in lake sediments: Journal of Paleolimnology, v. 21, p. 375–393, doi: 10.1023/A:1008066118210.
- Flusche, M., Seltzer, G., Rodbell, D., Siegel, D., and Samson, S., 2005, Constraining water sources and hydrologic processes from the isotopic analysis of water and dissolved strontium, Lake Junín, Peru: Journal of Hydrology (Amsterdam), v. 312, p. 1–13, doi: 10.1016/j.jhydrol.2005.02.021.
- Gurmendi, A.C., 2006, The Mineral Industry of Peru: USGS Minerals Yearbook: U.S. Geological Survey, 14 p., http://minerals.usgs.gov/minerals/pubs/country/2006/myb3-2006-pe.pdf (last accessed 3 Mar. 2014).
- Hunefeldt, C., 2004, A Brief History of Peru: New York, Checkmark Books and Lexington Associates, 304 p.
- Klarén, P.F., 2000, Peru: Society and Nationhood in the Andes: Oxford, UK, Oxford University Press, 512 p.
- Mark, B.G., McKenzie, J.M., and Gomez, J., 2005, Hydrochemical evaluation of changing glacier meltwater contribution to stream discharge, Callejon de Huayllas, Peru: Hydrological Sciences, v. 50, p. 975–987, doi: 10.1623/hysj.2005.50.6.975.
- Martin, A.J., McNee, J.J., and Pedersen, T.F., 2001, The reactivity of sediments impacted by metal-mining in Lago Junín, Peru: Journal of Geochemical Exploration, v. 74, p. 175–187, doi: 10.1016/S0375-6742(01)00183-2.
- Ministerio del Ambiente, 2010, Decreto Supremo Nº 010–2010-MINAM: Aprueban Límites Máximos Permisibles para la descarga de efluentes líquidos de actividades mineras-metalúrgicas: El Peruano, Normas Legales, p. 424,114–424,117.
- Pedersen, T.F., Martin, A.J., and McNee, J.J., 1999, Contrasting trace metal dynamics in contaminated Lago Junín Peru and submerged tailings deposits in Canadian lakes: The importance of permanent submergence: Proceedings of the International Conference on Mining and the Environment II, p. 165–175.
- Seltzer, G., Rodbell, D., and Burns, S., 2000, Isotopic evidence for late Quaternary climatic change in tropical South America: Geology, v. 28, p. 35–38, doi: 10.1130/0091-7613(2000)28<35:IEFLQC>2.0.CO;2.
- Shoobridge, D., 2006, Protected area profile: Peru: Junín National Reserve: ParksWatch, 37 p., http://www.parkswatch.org/parkprofiles/pdf/jnar_eng.pdf (last accessed 3 Mar. 2014).
- U.S. EPA, 1993, Clean Water Act: Washington, D.C., U.S. Environmental Protection Agency, section 503, v. 58, no. 32.
- U.S. EPA, 2009, National Primary Drinking Water Regulations: U.S. Environmental Protection Agency, EPA-816-F-09–004, May 2009, 6 p., http://www.epa.gov/safewater/consumer/pdf/mcl.pdf (last accessed 3 Mar. 2014).
- Veliz, C.Y., 2001, Anthropogenic and climatic impacts in the central Peruvian Andes: Evidence from the study of three tropical lakes [M.S. thesis]: Syracuse, New York, Syracuse University, 342 p.
- Verschuren, D., 1993, A lightweight extruder for accurate sectioning of soft-bottom lake sediment cores in the field: Journal of Paleolimnology, v. 38, p. 1796–1802.
- Volcan, 2013, Operations and Production Units: Volcan Compañia Minera, www.volcan.com.pe, July 2013 (last accessed 3 Mar. 2014).
- Vuille, M., Francou, B., Wagnon, P., Juen, I., Kaser, G., Mark, B.G., and Bradley, R.S., 2008, Climate change and tropical Andean glaciers: Past, present and future: Earth-Science Reviews, v. 89, p. 79–96, doi: 10.1016/j.earscirev.2008.04.002.
- Vuille, M., Burns, S.J., Taylor, B.L., Cruz, F.W., Bird, B.W., Abbott, M.B., Kanner, L.C., Cheng, H., and Novello, V.F., 2012, A review of the South American monsoon history as recorded in stable isotopic proxies over the past two millennia: Climate of the Past Discussions, v. 8, p. 637–668, doi: 10.5194/cpd-8-637-2012.
- World Bank, 2013, World development indicators: Electricity production, sources, and access: http://wdi.worldbank.org/table/3.7 (last accessed 3 Mar. 2014).

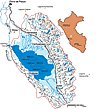 Figure 1
Figure 1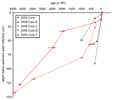 Figure 2
Figure 2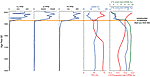 Figure 3
Figure 3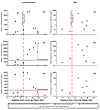 Figure 4
Figure 4 Table 1
Table 1