Full Text View
Volume 20 Issue 2 (February 2010)
GSA Today
Article, pp. 4-9 | Abstract | PDF (6.4MB)
Rock to regolith conversion: Producing hospitable substrates for terrestrial ecosystems
| Table of Contents |
|---|
| ERRATUM: On pages 7 and 8 of this article, rates listed as mm yr−1 and mm k.y. yr−1 were inadvertently changed to m yr−1 and m k.y.−1 — please note that the correct measurement is millimeters, not meters. |
|
Search GoogleScholar for
Search GSA Today |
Abstract
Weathering processes transform hard fresh rock into friable weathered rock, which is then physically disrupted to become soil. These regolith materials mantle the land masses and support terrestrial life but their formation involves some of the least understood of Earth’s surficial processes. The conversion of biologically inert hard rock to a hospitable substrate for organisms begins with the production of porosity by weathering. Porosity allows water to flow through weathered rock, but it also imparts a water-holding capacity so that water can be stored for prolonged use by organisms. Organisms themselves, in the form of microbes and plant roots, invade the rock as porosity forms. Production of porosity is the fundamental process responsible for converting rock into a medium capable of supporting terrestrial ecosystems. Consequently, the rate of porosity formation during rock weathering is the ultimate measure of the production and sustainability of ecosystem-functional substrates.
Manuscript received 22 May 2009; accepted 15 September 2009
DOI: 10.1130/GSAT57A.1
E-mail: , ,
INTRODUCTION
Fresh bedrock exposed at the land surface is an inhospitable substrate for most life. Exposed bedrock has very low porosity and hydraulic conductivity (Zhao, 1998; Schild et al., 2001); consequently, rain and snowmelt run off from it immediately. Water is not stored, so plants do not have a reservoir from which they can extract moisture as needed during dry periods. Furthermore, although hard bedrock contains elements such as P, Ca, Mg, and K that are essential for life, they are not readily accessible to organisms because they are bound within crystalline mineral structures. Once hard rock is weathered, it develops abundant porosity, first as friable bedrock, and later, when this weathered bedrock is physically disrupted, as soil. The development of extensive porosity is the key process in converting rock from a biologically inert material to a medium from which biota can gain nutrients, stored water, and a vast underground habitat. Here we describe the mechanisms and implications of transforming nonporous hard rock into porous regolith. We focus on granitic rock because it is a major component of Earth’s crust (15% of the land area) and because it is relatively consistent in its weathering behavior (Twidale and Vidal Romaní, 2005).
POROSITY FORMATION AND GRANITIC ROCK WEATHERING
Unweathered granitic plutons are commonly jointed. The joints are the result of stresses on the rock mass, including those associated with thermal, tectonic, and erosional unloading processes. Joint spacings range from several decimeters to several meters, can be orthogonally oriented, and depend on the geologic history of the rock. In unweathered bedrock, the joint fractures are empty planar voids that range in width from a fraction of a millimeter to more than a centimeter (Bergbauer and Martel, 1999). Fractures are the main source of hydraulic connectivity in unweathered bedrock (Paillet, 1993). The rock mass between the joints contains minor porosity, usually 1% or less (Twidale and Vidal Romaní, 2005), in the form of microfractures <1 μm wide and microporosity within mineral grains (Sardini et al., 2006). The microfractures are generated by stresses incurred during cooling, hydrothermal activity, or tectonism (Schild et al., 2001). Micropores within mineral grains form during crystallization and cooling. Meteoric water flowing down joint fractures initially enters the bedrock mass through inherent microfractures, thereby beginning the chemical weathering process (Meunier et al., 2007).
In biotite-bearing granites, ion exchange weathering is an important first step in generating bulk rock porosity. The replacement of interlayer K by hydrated Mg cations results in expansion of the biotite structure as the mineral is transformed to vermiculite (Wahrhaftig, 1965; Nettleton et al., 1970; Isherwood and Street, 1976). This expansion, which involves a 30%–40% increase in volume, exploits the weakness imparted by the lithogenic microfractures and shatters the rock. A smaller expansion of biotite has been noted to occur upon oxidation of the Fe within its structure (Buss et al., 2008). In either case, the rock matrix loses much of its mechanical strength (Arel and Önalp, 2004) and is transformed into a regolith material referred to as saprock (Anand and Paine, 2002) (Fig. 1A). The rock mass is now permeated by a continuous network of mesofractures (Fig. 1B). It maintains the original rock texture (Fig. 1C) but is friable and can be crumbled by hand into its individual grain sizes (Fig. 1D). Individual mineral grains in saprock are not extensively chemically altered (Wahrhaftig, 1965; Girty et al., 2003).
|
(A) Saprock in the central Sierra Nevada, California. Note graffiti easily carved into this friable bedrock material (tile spade for scale: 1.15 m). (B) Thin section micrograph (plane light) showing porosity (in blue) and partially weathered biotite (b) and plagioclase (p). Primary minerals predominate, and very little clay has been produced by weathering. Saprock maintains rock texture (C), but is easily crushed in bare hands (D). |
The mesofracture network in saprock opens up the rock mass to extensive percolation of water and vastly increases the surface area for weathering. At this point, hydrolysis becomes an effective weathering process, attacking feldspars and other weatherable minerals. Feldspars are weathered preferentially along twin planes (Fig. 1B), and are eventually pseudomorphically replaced by kaolin or gibbsite (Inskeep et al., 1993; Taboada and García 1999; Jiménez-Espinosa et al., 2007). The highly weathered bedrock mass, termed saprolite, still retains rock texture (Fig. 2A), but most weatherable minerals, such as feldspars, micas, and amphiboles, are altered to clay minerals (Fig. 2B). Saprolite can be crumbled by hand and is plastic when wet (Fig. 2C). New sources of porosity in saprolite are produced in the form of dissolution pits in relict primary minerals and as interstitial pores within masses of precipitated clay minerals (Frazier and Graham, 2000; White et al., 2001; Turner et al., 2003).
|
(A) Saprolite on the North Carolina Piedmont. Rock structural features are preserved, yet saprolite is soft and easily excavated. (Note bulldozer for scale, lower right; photo credit: G. Simpson). (B) Thin section micrograph (cross-polarized light) showing thorough alteration of weatherable primary minerals to clay minerals (photo credit: M. Vepraskas). (C) Due to this extensive weathering and clay production, saprolite is plastic when wet. |
HYDRAULIC BEHAVIOR OF WEATHERED ROCK
When bedrock has been weathered to saprock, joint traces remain distinct (Fig. 3A) but are wider and filled with a sandy loam material that has been dislodged from the joint walls (Sternberg et al., 1996; Graham et al., 1997). These joint fractures in saprock are pathways for rapid preferential movement of water (Fig. 3B) (Frazier et al., 2002), but fractures in saprolite can become plugged with translocated materials such as clay and iron and manganese oxides, diminishing their ability to transmit water (Schoeneberger and Amoozegar, 1990; Vepraskas, 2005). Mesofractures between joints in saprock are sufficiently wide to allow gravitational flow of water, but they present a tortuous path for water flow (Fig. 3C), resulting in a lower hydraulic conductivity than the joints (Frazier et al., 2002). Clay produced by weathering is translocated in suspension and deposited on mesofracture walls (Fig. 3D) (Graham et al., 1994; Frazier and Graham, 2000). Dissolution pits in primary minerals and interstitial pores in the clay materials are micropores (<10 μm diameter), so they retain water against the force of gravity (Luxmore, 1981). Thus, as primary minerals are altered, the weathered rock gains the ability to store appreciable amounts of water (Jones and Graham, 1993; Hubbert et al., 2001b). Below a depth of several decimeters, this water is not readily lost by evaporation and is available to support plants during the dry season.
|
Illustration of porosity types in granitic saprock of the San Jacinto Mountains, California. (A) Joint fractures bound matrix blocks. (B) Joint fractures stained by preferential flow of blue dye tracer; note wetting front in matrix lags behind that in joints (photo credit: S. Frazier). (C) Thin section micrograph (plane light) of saprock matrix showing porosity in blue. Note mesofractures, partially expanded biotite (b), and dissolution-pitted plagioclase (dp). (D) Close-up of outlined area in (C), showing dissolution pitting (dp) following albite twin planes and clay films (cf) lining the mesofracture wall. |
BIOLOGICAL ACCESS
Terrestrial plants require a porous substrate in order for their roots to gain structural support and to access water and nutrients. The roots transfer water and nutrients to the above-ground part of the ecosystem and return photosynthetically fixed atmospheric carbon to the belowground part of the ecosystem in the form of root biomass. The roots provide energy to a multitude of soil organisms, promote further weathering, and physically alter regolith morphology (Graham et al., 1994; Frazier and Graham, 2000; Schenk and Jackson, 2005).
Depth of rooting is directly related to climate. Woody plants that experience seasonal drought have roots that extend deep into the substrate to access stored water (Schenk and Jackson, 2002). When bedrock occurs within this potential rooting depth, plant roots penetrate below the soil into fractures in the rock. This phenomenon is common in upland areas where thin soils (<1 m thick) overlie bedrock. For example, roots of ponderosa pine seedlings reach the subsoil saprock within their first two years in the central Sierra Nevada, California (Witty et al., 2003). Mature ponderosa pine roots can extend 24 m deep into fractured bedrock, and juniper roots can go much deeper (>61 m) (Stone and Kalisz, 1991). Roots of chaparral shrubs (Sternberg et al., 1996), oaks (Bornyasz et al., 2005), and conifers (Anderson et al., 1995; Hubbert et al., 2001a, 2001b) extend deeper than 4 m into saprock in the mountains of California.
Plant roots grow along paths of least resistance, so they follow fractures in bedrock. Even in saprock, roots remain confined to joint fractures, forming dense root mats (Sternberg et al., 1996; Hubbert et al., 2001b; Bornyasz et al., 2005). On the other hand, the porous rock matrix is where the water is stored. Because roots are confined to joint fractures and the water is stored in the matrix between the fractures, there must be a mechanism by which water is moved from the center of the weathered matrix block toward the fractures, a distance of 0.25–0.5 m. Water might move via unsaturated flow along a moisture potential gradient set up by the roots. However, the unsaturated hydraulic conductivity of saprock is very low. We estimate that, at water potentials of <−0.1 MPa, unsaturated flow occurs at a rate of <10−3 cm h−1 (Hubbert et al., 2001b). Hence, more than a year would be required for water to move from the center of a matrix block to the joint fractures. This exceeds the length of the dry season and does not explain the annual depletion of water observed in saprock (Arkley, 1981; Sternberg et al., 1996; Hubbert et al., 2001a; Bornyasz et al., 2005).
The gap between root occurrence in fractures and water storage in the weathered rock matrix is bridged by a symbiosis between plants and fungi. The roots of wildland trees and shrubs are infected with mycorrhizal fungi in a symbiotic relationship in which the fungus obtains carbon from, and delivers water and nutrients to, the root (Allen, 2007). Mycorrhizal fungi have hyphae that extend more than a meter from the host root and are <20 μm in diameter; thus, they can easily explore meso-fractures in the saprock matrix (Fig. 4). In the process, mycorrhizal hyphae may promote biotite weathering (Balogh-Brunstad et al., 2008), the critical first step in saprock production. The presence of mycorrhizal hyphae as deep as four meters within the saprock matrix under oak woodland (Bornyasz et al., 2005) and chaparral (Egerton-Warburton et al., 2003) suggests that water is being tapped from the capillary-size pores.
|
Scanning electron micrograph of mycorrhizal fungal hyphae penetrating a saprock microfracture between feldspar (fsp), quartz (q), and partially kaolinized feldspar (k) grains. Scale bar: 100 μm. Sample was taken from the 40-cm depth in saprock shown in Figure 3A. |
SUPPORT FOR ECOSYSTEMS
In upland granitic terrain in California, thin soils overlie a thick zone of saprock. Although soil has a greater water-
holding capacity, the saprock, because of its greater thickness, constitutes the greatest reservoir of plant-available water. For example, in a Jeffrey pine forest in the southern Sierra Nevada, the regolith consists of an upper 75-cm-thick layer of soil with a plant-available water capacity (PAWC) of 20% that overlies a 275-cm-thick layer of saprock with a PAWC of 12%. The result is that the soil retains 15 cm of water in its 75 cm thickness, whereas the saprock holds more than twice this amount (33 cm). Since this forest site loses at least 40 cm of water by evapotranspiration annually, mostly during the summer dry season, water stored within the soil cannot support the water demands of the forest (Rose et al., 2003). For example, in 1996, plant-available water in the soil was depleted by the end of June (Fig. 5), and the plants had to rely on water stored within the saprock for the remainder of the summer dry season (which extended to the end of October).
|
Plant-available water of the soil and saprock zones under a Jeffrey pine forest in the southern Sierra Nevada, California, as a function of time during the dry season of 1996. Note plant-available water was depleted from the soil zone by the end of June. For the remainder of the dry season, forest vegetation relied on water stored in the saprock. |
In arid and semi-arid regions, water availability is the major limitation to plant growth, whereas mineral-derived nutrients such as Ca, Mg, K, and P are generally present in sufficient quantities (e.g., Hubbert et al., 2001b). In these regions, weathered bedrock benefits ecosystems primarily by increasing the water-storage volume beyond that provided by the overlying soil. In more humid regions, soils are usually moist, so water availability is not limiting, but mineral-derived nutrients are often depleted by leaching (e.g., Oh and Richter, 2005) or are specifically adsorbed to Fe- or Al-oxide weathering products and unavailable for plant uptake (Buol et al., 2003). In such cases, weathered rock may benefit the ecosystem by supplying nutrients to plants whose roots and symbiotic fungi reach bedrock or exploit rock fragments in the soil (Ugolini et al., 2001; Heisner et al., 2004).
SOIL SUSTAINABILITY
Soils are recognized as the foundation for terrestrial ecosystems (Doran and Parkin, 1994) and are a major factor in ecosystem and agricultural sustainability (Montgomery, 2007). But what should we consider to be “soil”? While traditional views hold that soil lacks rock structure (Soil Survey Staff, 1999), and a “soil production function” has been developed based on the rate of disruption of weathered bedrock (Heimsath et al., 1999, 2000), weathered bedrock (saprock and saprolite) itself functions much like soil in an ecosystem and hydrologic sense. The rate at which functional substrate for plants is produced is determined by the rate of porosity formation during rock weathering. This is particularly true for ecosystems in which weathered bedrock is a component of the water storage reservoir that is heavily drawn upon during dry seasons. If the rate of soil erosion exceeds the rate of porosity formation, the existing ecosystem is not sustainable (Fig. 6A). Therefore, the rate at which hard rock is converted to porous saprock is the appropriate measure of the production and sustainability of ecosystem-functional substrate.
|
(A) Bedrock weathering fronts move inward from fractures. The entire vadose zone is subjected to weathering processes. From an ecosystem-sustainabili-ty standpoint, functional substrate thickness is maintained when erosional losses are balanced by saprock production. (B) Weathered rock profile showing joint fractures and less-weathered corestones at the center of joint blocks. Below arrow on right, note roots along fracture plane where corestone has fallen out. |
The rate of subsurface rock weathering has been addressed from a geochemical view (e.g., Colman and Dethier, 1986; Brantley et al., 2008; Burke et al., 2009), but less emphasis has been placed on the rate of porosity formation. By studying granitic clast weathering in moraines of the Sierra Nevada, California, Rossi and Graham (2010) determined that 10-cm-diameter clasts were altered to saprock only in those moraines older than 81 ka. These clasts held plant-available water and hosted mycorrhizal fungal hyphae (i.e., they were functioning as part of the ecosystem substrate). We use this observation to estimate the rate of ecosystem-functional substrate production from granitic bedrock.
Because the weathering front moves inward from all sides of the clasts, the clast weathering profile is best approximated as the radius. If we assume the clasts to be spherical, this corresponds to a radius of 5 cm. In other words, in 81 k.y., a rock thickness of 5 cm has been transformed to ecosystem substrate (Rossi and Graham, 2010). This is equivalent to 0.6 m k.y.−1 of weathering front movement. In contrast, saprolite production from granodiorite in southeastern Australia ranges from 4 to 46 m k.y.−1, depending on landscape position (Dosseto et al., 2008). While higher weathering rates in southeastern Australia may be expected due to a higher mean annual precipitation (910 m yr−1) than the Sierra Nevada site (200 m yr−1), the manner in which weathering occurs also needs to be considered for this comparison.
Weathering fronts in granitic bedrock are not smooth planar features (Fig. 6B). Instead, they consist of a zone defined by the depth to which meteoric water penetrates (i.e., the vadose zone). Within this zone, bedrock blocks are weathered from all sides by water percolating through joint fractures (Fig. 6A). The vadose zone in residual profiles of granitic rock in the southern Sierra Nevada and the Peninsular Ranges in California is commonly 4–8 m deep (Hellmers et al., 1955; Hubbert et al., 2001a) with joint spacings of 50 cm (Wahrhaftig, 1965; Sternberg et al., 1996; Witty et al., 2003). Based on the granitic clast weathering rates determined by Rossi and Graham (2010), hard granitic bedrock with joints spaced 50 cm apart could be weathered to saprock in ~400 k.y. The rate of saprock production would be 0.01 m yr−1 if the rock weathering zone was 4 m thick, or 0.02 m yr−1 if it was 8 m thick. These rates (0.01–0.02 m yr−1) assume simultaneous weathering throughout the vadose zone and are similar to the southeastern Australia saprolite production rates (0.004–0.046 m yr−1) (Dosseto et al., 2008).
If erosion rates exceed the rate of saprock production (Fig. 6A), the substrate (soil plus saprock) is not sustainable, and consequently neither is the ecosystem. Erosion rates in granitic terrain of the northern Sierra Nevada range from 0.015 to 0.06 m yr−1, with higher rates on steeper slopes (Granger et al., 2001). Our calculated rates of saprock production (0.01–0.02 m yr−1) are the same magnitude as the erosion rate, implying that the regolith has attained an equilibrium thickness on stable landscape positions but is depleted on steep slopes.
CONCLUSIONS
Porosity produced by weathering converts biologically inert rock into a material that supplies organisms with habitat, stored water, and nutrients. Initial weathering of granitic rock produces saprock, which retains rock texture and fresh primary minerals, but has an extensive network of mesofractures, is friable, and holds plant-available water. Roots are confined to and fully occupy joint fractures to at least 4 m in depth. Matrix water is delivered to them via mycorrhizal fungal hyphae that explore the mesofractures. Further eathering produces saprolite, which is plastic when wet, has abundant capillary-size pores in clay masses and dissolution-pitted primary minerals, and holds more water than saprock. Deep-rooted trees and shrubs rely on water stored in weathered bedrock to survive summer drought. Because these porous rock materials function intimately in terrestrial ecosystems, the rate of porosity formation during rock weathering is the appropriate measure of ecosystem-functional substrate production.
ACKNOWLEDGMENTS
Preparation of this paper was supported in part by funding from the University of California Kearney Foundation of Soil Science. We thank Krassimir Bozhilov for scanning electron microscopy and Mike Allen for comments on the fungal hyphae (Fig. 4). Reviews by Douglas Morton, Ronald Amundson, and Marjorie Schulz, and editing by Stephen Johnston, helped improve the paper.
REFERENCES CITED
- Allen, M.F., 2007, Mycorrhizal fungi: Highways for water and nutrients in arid soils: Vadose Zone Journal, v. 6, p. 291–297, doi: 10.2136/vzj2006.0068.
- Anand, R.R., and Paine, M., 2002, Regolith geology of the Yilgarn Craton, western Australia: Implications for exploration: Australian Journal of Earth Sciences, v. 49, p. 3–162, doi: 10.1046/j.1440-0952.2002.00912.x.
- Anderson, M.A., Graham, R.C., Alyanakian, G.J., and Martynn, D.Z., 1995, Late summer water status of soils and weathered bedrock in a giant sequoia grove: Soil Science, v. 160, p. 415–422, doi: 10.1097/00010694-199512000-00007.
- Arel, E., and Önalp, A., 2004, Diagnosis of the transition from rock to soil in a granodiorite: Journal of Geotechnical and Geoenvironmental Engineering, v. 130, p. 968–974, doi: 10.1061/(ASCE)1090-0241(2004)130:9(968).
- Arkley, R.J., 1981, Soil moisture use by mixed confer forest in a summer-dry climate: Soil Science Society of America Journal, v. 45, p. 423–427.
- Balogh-Brunstad, Z., Keller, C.K., Dickinson, J.T., Stevens, F., Li, C.Y., and Bormann, B.T., 2008, Biotite weathering and nutrient uptake by ectomycorrhizal fungus, Suillus tomentosus, in liquid-culture experiments: Geochimica et Cosmochimica Acta, v. 72, p. 2601–2618, doi: 10.1016j.gca.2008.04.003.
- Bergbauer, S., and Martel, S.J., 1999, Formation of joints in cooling plutons: Journal of Structural Geology, v. 21, p. 821–835, doi: 10.1016/S0191-8141(99)00082-6.
- Bornyasz, M.A., Graham, R.C., and Allen, M.F., 2005, Ectomycorrhizae in a soil-weathered granitic bedrock regolith: Linking matrix resources to plants: Geoderma, v. 126, p. 141–160, doi: 10.1016/j.geoderma.2004.11.023.
- Brantley, S.L., Kubicki, J.D., and White, A.F., editors, 2008, Kinetics of Water-Rock Interaction: Berlin, Springer, 840 p.
- Buol, S.B., Southard, R.J., Graham, R.C., and McDaniel, P.A., 2003, Soil Genesis and Classification: Ames, Iowa State Press, 494 p.
- Burke, B.C., Heimsath, A.M., Dixon, J.L., Chappell, J., and Yoo, K., 2009, Weathering the escarpment: Chemical and physical rates and processes, south-eastern Australia: Earth Surface Processes and Landforms, v. 34, p. 768–785, doi: 10.1002/esp.1764.
- Buss, H.L., Sak, P.B., Webb, S.M., and Brantley, S.L., 2008, Weathering of the Rio Blanco quartz diorite, Luquillo Mountains, Puerto Rico: Coupling oxidation, dissolution, and fracturing: Geochimica et Cosmochimica Acta, v. 72, p. 4488–4507, doi: 10.1016/j.gca.2008.06.020.
- Colman, S.M., and Dethier, D.P., eds., 1986, Rates of Chemical Weathering of Rocks and Minerals: Orlando, Academic Press, 603 p.
- Doran, J.W., and Parkin, T.B., 1994, Defining and assessing soil quality, in Doran, J.W., Coleman, D.C., Bezdicek, D.F., and Stewart, B.A., eds., Defining Soil Quality for a Sustainable Environment: Soil Science Society of America Special Publication Number 35, p. 3–21.
- Dosseto, A., Turner, S.P., and Chappell, J., 2008, The evolution of weathering profiles through time: New insights from uranium-series isotopes: Earth and Planetary Science Letters, v. 274, p. 359–371, doi: 10.1016/j.epsl.2008.07.050.
- Egerton-Warburton, L.M., Graham, R.C., and Hubbert, K.R., 2003, Spatial variability in mycorrhizal hyphae and nutrient and water availability in a soil-weathered bedrock profile: Plant and Soil, v. 249, p. 331–342, doi: 10.1023/A:1022860432113.
- Frazier, C.S., and Graham, R.C., 2000, Pedogenic transformation of fractured granitic bedrock, southern California: Soil Science Society of America Journal, v. 64, p. 2057–2069.
- Frazier, C.S., Graham, R.C., Shouse, P.J., Yates, M.V., and Anderson, M.A., 2002, A field study of water flow and virus transport in weathered granitic bedrock: Vadose Zone Journal, v. 1, p. 113–124, doi: 10.2113/1.1.113.
- Girty, G.H., Marsh, J., Meltzner, A., McConnell, J.R., Nygren, D., Nygren, J., Prince, G.M., Randall, K., Johnson, D., Heitman, B., and Nielsen, J., 2003, Assessing changes in elemental mass as a result of chemical weathering of granodiorite in a Mediterranean (hot summer) climate: Journal of Sedimentary Research, v. 73, p. 434–443, doi: 10.1306/091802730434.
- Graham, R.C., Guertal, W.R., and Tice, K.R., 1994, The pedologic nature of weathered rock, in Cremeens, D.L., Brown, R.B., and Huddleston, J.H., eds., Whole Regolith Pedology: Soil Science Society of America Special Publication Number 34, p. 21–40.
- Graham, R.C., Schoeneberger, P.J., Anderson, M.A., Sternberg, P.D., and Tice, K.R., 1997, Morphology, porosity, and hydraulic conductivity of weathered granitic bedrock and overlying soils: Soil Science Society of America Journal, v. 61, p. 516–522.
- Granger, D.E., Riebe, C.S., Kirchner, J.W., and Finkel, R.C., 2001, Modulation of erosion on steep granitic slopes by boulder armoring, as revealed by cosmogenic 26Al and 10Be: Earth and Planetary Science Letters, v. 186, p. 269–281, doi: 10.1016/S0012-821X(01)00236-9.
- Heimsath, A.R., Dietrich, W.E., Nishiizumi, K., and Finkel, R.C., 1999, Cosmogenic nuclides, topography, and the spatial variation of soil depth: Geomorphology, v. 27, p. 151–172, doi: 10.1016/S0169-555X(98)00095-6.
- Heimsath, A.R., Chappell, J., Dietrich, W.E., Nishiizumi, K., and Finkel, R.C., 2000, Soil production on a retreating escarpment in southeastern Australia: Geology, v. 28, p. 787–790, doi: 10.1130/0091-7613(2000)28<787:SPOARE>2.0.CO;2.
- Heisner, U., Raber, B., and Hildebrand, E.E., 2004, The importance of the soil skeleton for plant-available nutrients in sites of the Southern Black Forest, Germany: European Journal of Forest Research, v. 123, p. 249–257, doi: 10.1007/s10342-004-0041-7.
- Hellmers, H., Horton, J.S., Juhren, G., and O’Keefe, J., 1955, Root systems of some chaparral plants in southern California: Ecology, v. 36, p. 667–678, doi: 10.2307/1931305.
- Hubbert, K.R., Beyers, J.L., and Graham, R.C., 2001a, Roles of weathered bedrock and soil in seasonal water relations of Pinus jeffreyi and Arctostaphylos patula: Canadian Journal of Forest Research, v. 31, p. 1947–1957, doi: 10.1139/cjfr-31-11-1947.
- Hubbert, K.R., Graham, R.C., and Anderson, M.A., 2001b, Soil and weathered bedrock: Components of a Jeffrey pine plantation substrate: Soil Science Society of America Journal, v. 65, p. 1255–1262.
- Inskeep, W.P., Clayton, J.L., and Mogk, D.W., 1993, Naturally weathered plagioclase grains from the Idaho Batholith: Observations using scanning electron microscopy: Soil Science Society of America Journal, v. 57, p. 851–860.
- Isherwood, D., and Street, A., 1976, Biotite-induced grussification of the Boulder Creek granodiorite, Boulder County, Colorado: Geological Society of America Bulletin, v. 87, p. 366–370, doi: 10.1130/0016-7606(1976)87<366:BGOTBC>2.0.CO;2.
- Jiménez-Espinosa, R., Vázquez, M., and Jiménez-Millán, J., 2007, Differential weathering of granitic stocks and landscape effects in a Mediterranean climate, Southern Iberian Massif (Spain): CATENA, v. 70, p. 243–252, doi: 10.1016/j.catena.2006.09.001.
- Jones, D.P., and Graham, R.C., 1993, Water-holding characteristics of weathered granitic rock in chaparral and forest ecosystems: Soil Science Society of America Journal, v. 57, p. 256–261.
- Luxmore, R.J., 1981, Micro-, meso-, and macroporosity of soil: Soil Science Society of America Journal, v. 45, p. 671.
- Meunier, A., Sarsdini, P., Robinet, J.C., and Prêt, D., 2007, The petrography of weathering processes: facts and outlooks: Clay Minerals, v. 42, p. 415–435, doi: 10.1180/claymin.2007.042.4.01.
- Montgomery, D.R., 2007, Is agriculture eroding civilization’s foundation?: GSA Today, v. 17, p. 4–9, doi: 10.1130/GSAT01710A.1.
- Nettleton, W.D., Flach, K.W., and Nelson, R.E., 1970, Pedogenic weathering of tonalite in southern California: Geoderma, v. 4, p. 387–402, doi: 10.1016/0016-7061(70)90055-8.
- Oh, N.-H., and Richter, D.D., 2005, Elemental translocation and loss from three highly weathered soil-bedrock profiles in the southeastern United States: Geoderma, v. 126, p. 5–25, doi: 10.1016/j.geoderma.2004.11.005.
- Paillet, F.L., 1993, Using borehole geophysics and cross-borehole flow testing to define hydraulic connections between fracture zones in bedrock aquifers: Journal of Applied Geophysics, v. 30, p. 261–279, doi: 10.1016/0926-9851(93)90036-X.
- Rose, K.L., Graham, R.C., and Parker, D.R., 2003, Water source utilization by Pinus jeffreyi and Arctostaphylos patula on thin soils over bedrock: Oecologia, v. 134, p. 46–54, doi: 10.1007/s00442-002-1084-4.
- Rossi, A.M., and Graham, R.C., 2010, Weathering and porosity formation in sub-soil granitic clasts, Bishop Creek moraines, California: Soil Science Society of America Journal (in press).
- Sardini, P., Siitari-Kauppi, M., Beaufort, D., and Hellmuth, K.-H., 2006, On the connected porosity of mineral aggregates in crystalline rocks: The American Mineralogist, v. 91, p. 1069–1080, doi: 10.2138/am.2006.1939.
- Schenk, H.J., and Jackson, R.B., 2002, The global biogeography of roots: Ecological Monographs, v. 72, p. 311–328.
- Schenk, H.J., and Jackson, R.B., 2005, Mapping the global distribution of deep roots in relation to climate and soil characteristics: Geoderma, v. 126, p. 129–140, doi: 10.1016/j.geoderma.2004.11.018
- Schild, M., Siegesmund, S., Vollbrect, A., and Mazurek, M., 2001, Characterization of granite matrix porosity and pore-space geometry by in situ and laboratory methods: Geophysical Journal International, v. 146, p. 111–125, doi: 10.1046/j.0956-540x.2001.01427.x.
- Schoeneberger, P., and Amoozegar, A., 1990, Directional saturated hydraulic conductivity and macropore morphology of a soil-saprolite sequence: Geoderma, v. 46, p. 31–49, doi: 10.1016/0016-7061(90)90005-T.
- Soil Survey Staff, 1999, Soil Taxonomy: Washington, D.C., U.S. Government Printing Office, 869 p.
- Sternberg, P.D., Anderson, M.A., Graham, R.C., Beyers, J.L., and Tice, K.R., 1996, Root distribution and seasonal water status in weathered granitic bedrock under chaparral: Geoderma, v. 72, p. 89–98, doi: 10.1016/0016-7061(96)00019-5.
- Stone, E.L., and Kalisz, P.J., 1991, On the maximum extent of tree roots: Forest Ecology and Management, v. 46, p. 59–102, doi: 10.1016/0378-1127(91)90245-Q.
- Taboada, T., and García, C., 1999, Pseudomorphic transformation of plagioclases during the weathering of granitic rocks in Galacia (NW Spain): CATENA, v. 35, p. 291–302, doi: 10.1016/S0341-8162(98)00108-8.
- Turner, B.F., Stallard, R.F., and Brantley, S.L., 2003, Investigation of in situ weathering of quartz diorite bedrock in the Rio Icacos basin, Luquillo Experimental Forest, Puerto Rico: Chemical Geology, v. 202, p. 313–341, doi: 10.1016/j.chemgeo.2003.05.001.
- Twidale, C.R., and Vidal Romaní, J.R., 2005, Landforms and Geology of Granitic Terrains: A.A. Balkema Publishers, Leiden, Netherlands, 351 p.
- Ugolini, F.C., Corti, G., Dufey, J.E., Agnelli, A., and Certini, G., 2001, Exchangeable Ca, Mg, and K of rock fragments and fine earth from sandstone and siltstone derived soils and their availability to grass: Journal of Plant Nutrition and Soil Science, v. 164, p. 309–315, doi: 10.1002/1522-2624(200106)164:3<309::AID-JPLN309>3.0.CO;2-8.
- Vepraskas, M.J., 2005, Predicting contaminant transport along quartz veins above the water table in a mica-schist saprolite: Geoderma, v. 126, p. 47–57, doi:10.1016/j.geoderma.2004.11.006.
- Wahrhaftig, C., 1965, Stepped topography of the southern Sierra Nevada, California: Geological Society of America Bulletin, v. 76, p. 1165–1190, doi: 10.1130/0016-7606(1965)76[1165:STOTSS]2.0.CO;2.
- White, A.F., Bullen, T.D., Schulz, M.S., Blum, A.E., Huntington, T.G., and Peters, N.E., 2001, Differential rates of feldspar weathering in granitic regoliths: Geochimica et Cosmochimica Acta, v. 65, p. 847–869, doi: 10.1016/S0016-7037(00)00577-9.
- Witty, J.H., Graham, R.C., Hubbert, K.R., Doolittle, J.A., and Wald, J.A., 2003, Contributions of water supply from the weathered bedrock zone to forest soil quality: Geoderma, v. 114, p. 389–400, doi: 10.1016/S0016-7061(03)00051-X.
- Zhao, J., 1998, Rock mass hydraulic conductivity of the Bukit Timah granite, Singapore: Engineering Geology, v. 50, p. 211–216, doi: 10.1016/S0013-7952(98)00021-0.

 Figure 1
Figure 1 Figure 2
Figure 2 Figure 3
Figure 3 Figure 4
Figure 4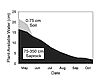 Figure 5
Figure 5